FDA APPROVES VIREAD® FOR CHRONIC HEPATITIS B
Leading Cause of Liver Cancer Affects 2 Million People in United States
The FDA has approved Viread® (tenofovir disoproxil fumarate) as a new treatment option for adults with chronic hepatitis B, a serious liver disease. Chronic hepatitis B affects an estimated 2 million people in the United States and is the leading cause of liver cancer worldwide.
For important safety information and full prescribing information, please visit www.Viread.com.
Manufacturing Footage
|
Viread packaging and manufacturing footage 
|
Research and Lab Footage
|
Gilead scientific research and lab footage 
|
Hi-Res Photo
|
Viread bottle 
|
Graphic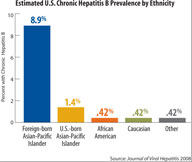
|
Bar chart of estimated chronic hepatitis B prevalence by ethnicity 
|
Related Document
|
Video Provided By: "Gilead Sciences"

Click here for more broadcast video & audio news releases and other source material from PR Newswire.
Assets For This Story
Hardcopy Request
Additional Documents
Download the following materials for this News Package:
Use the icons above for more information or call MultiVu Media Relations at